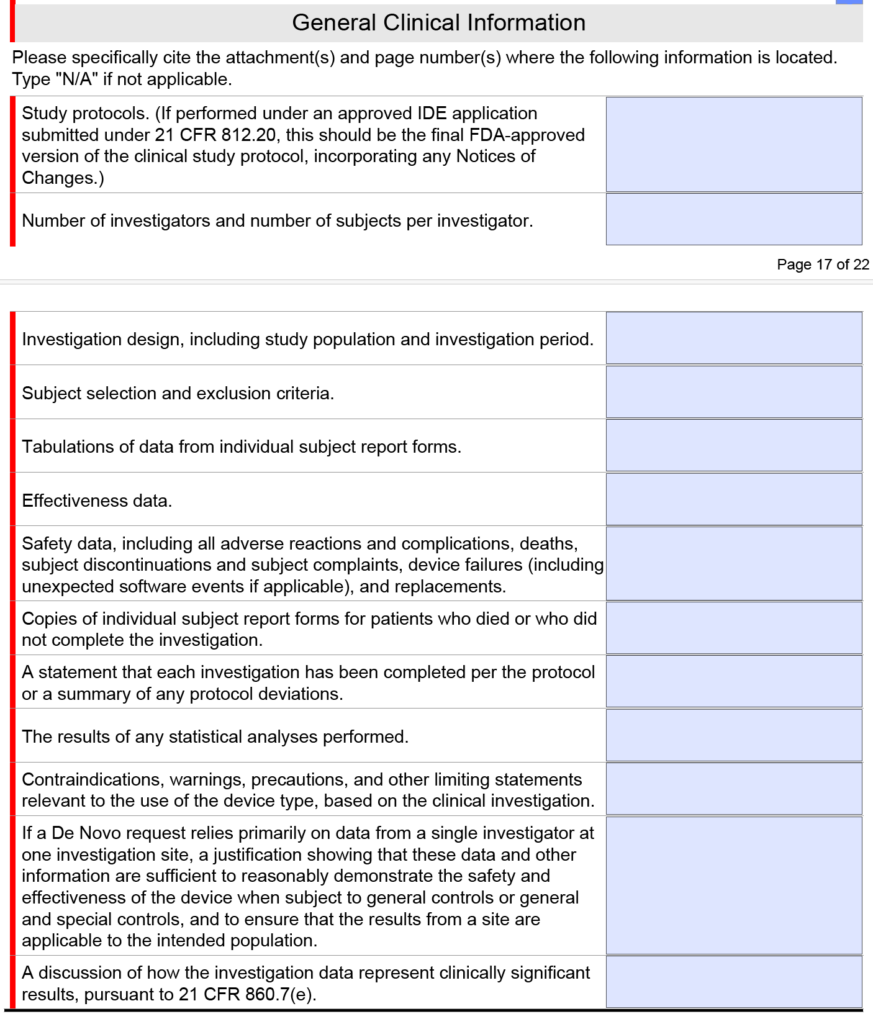
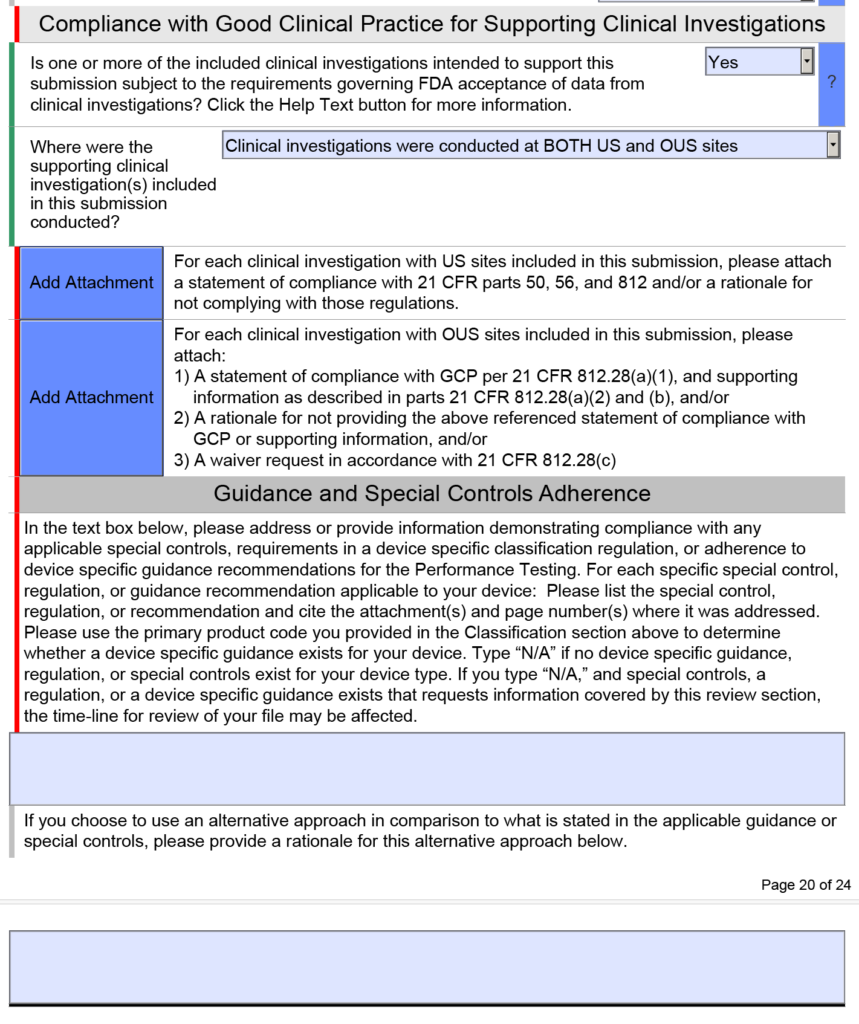
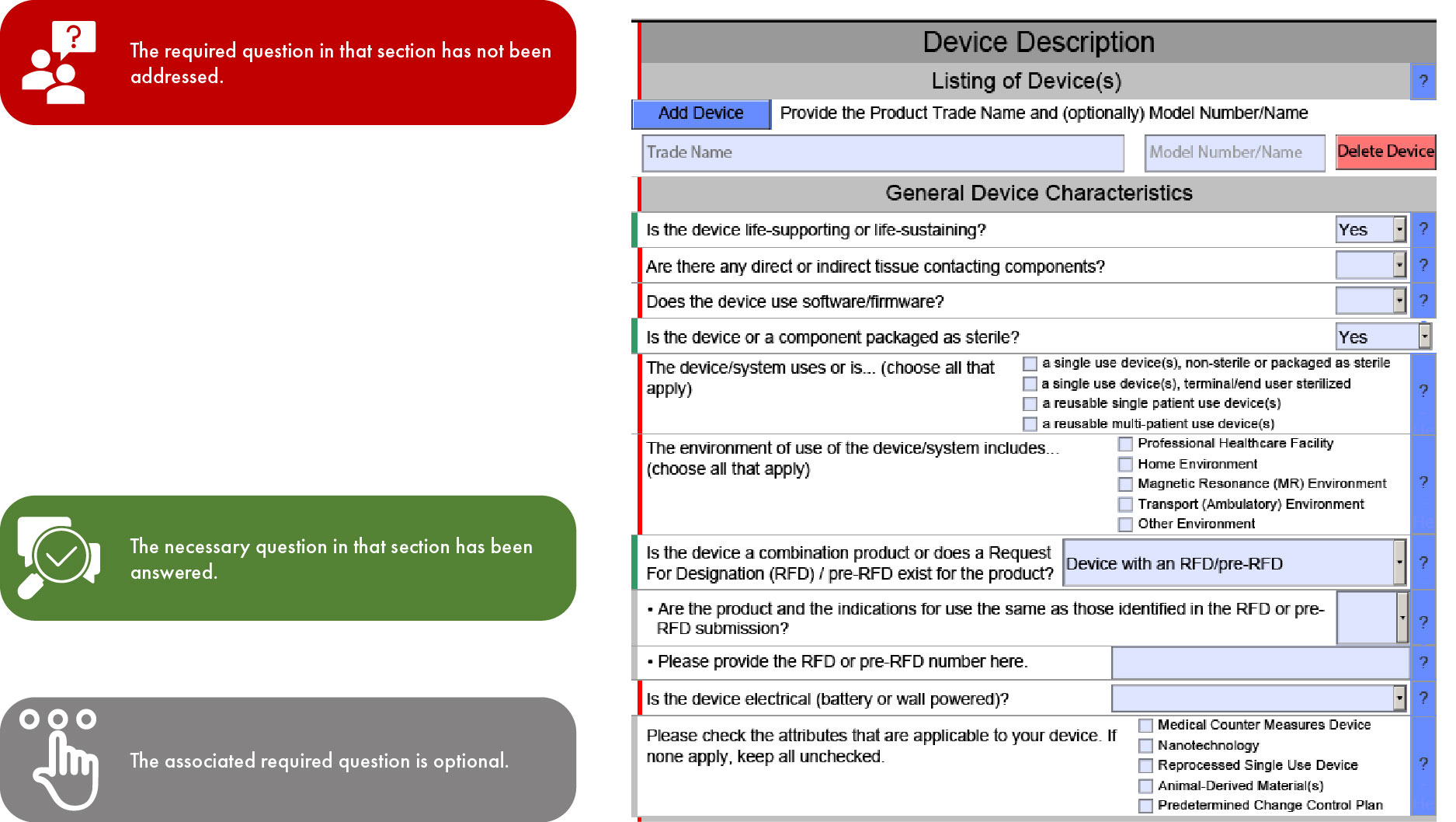
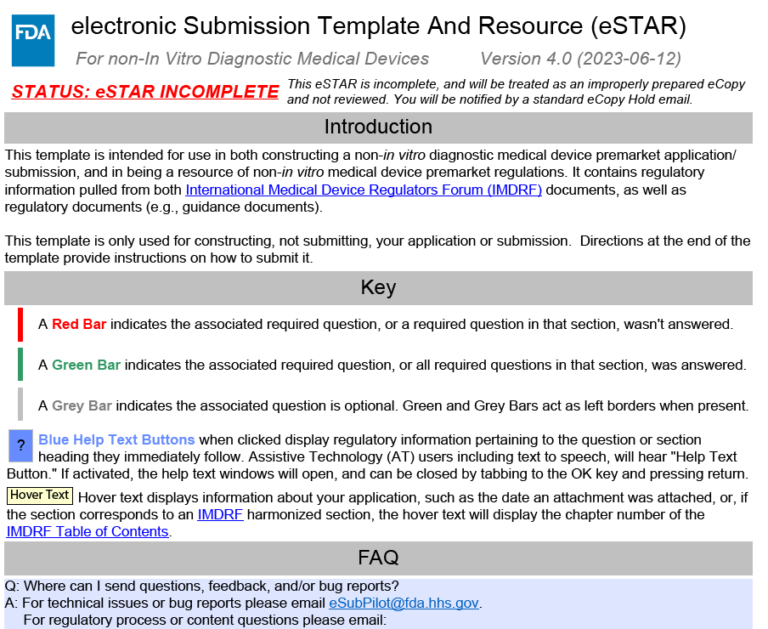
Fda Estar Template - Learn requirements, technical specs, compliance. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Complete guide to fda's mandatory estar electronic submission template. Estar (electronic submission template and resource): Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro.
Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Complete guide to fda's mandatory estar electronic submission template. Learn requirements, technical specs, compliance. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Estar (electronic submission template and resource): Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro.
Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Complete guide to fda's mandatory estar electronic submission template. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Estar (electronic submission template and resource): Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro. Learn requirements, technical specs, compliance.
About U.S FDA eSTAR eStarHelper
Learn requirements, technical specs, compliance. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro. Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Complete guide to fda's.
Using the new eSTAR templates for a 510(k) submission and the FDA eSTAR
Complete guide to fda's mandatory estar electronic submission template. Learn requirements, technical specs, compliance. Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Estar (electronic submission template and resource):
eSTAR Archives Medical Device Academy
Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro. Learn requirements, technical specs, compliance. Complete guide to fda's mandatory estar electronic submission template. Estar (electronic submission template and resource):
FDA eSTAR v5.5 What's new?
Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Learn requirements, technical specs, compliance. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Estar (electronic submission template and resource): Complete guide to fda's mandatory estar electronic submission template.
About U.S FDA eSTAR eStarHelper
Learn requirements, technical specs, compliance. Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Estar (electronic submission template and resource): Download the proper estar pdf template from the table below and save it before you open it in adobe.
FDA eSTAR Program, eSTAR Submissions, 510(k) Submission
Learn requirements, technical specs, compliance. Estar (electronic submission template and resource): Complete guide to fda's mandatory estar electronic submission template. Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro.
What is the FDA eSTAR program?
Learn requirements, technical specs, compliance. Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro. Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Complete guide to fda's.
FDA eSTAR Submission Template A Guide to Navigating the Process
Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Complete guide to fda's mandatory estar electronic submission template..
FDA eSTAR Submission Template A Guide to Navigating the Process
Learn requirements, technical specs, compliance. Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Complete guide to fda's.
FDA 510(k) Submissions to Use eSTAR Change in Submissions
Complete guide to fda's mandatory estar electronic submission template. Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Estar (electronic submission template and resource): Learn requirements, technical specs, compliance.
Estar (Electronic Submission Template And Resource):
Here’s what medical device manufacturers need to know about the electronic submission template and resource (estar) that. Download the proper estar pdf template from the table below and save it before you open it in adobe acrobat pro. Interactive pdf forms for 510(k), de novo, and presub submissions to cdrh aligns. Complete guide to fda's mandatory estar electronic submission template.