Fe Exam Equation Sheet - Iron makes up 5 percent of earth’s crust. Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. Iron has four stable isotopes: The fe exam is generally your first step in the process of becoming a licensed professional engineer (p.e.). The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. It is designed for recent graduates. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%).
54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%). The fe exam is generally your first step in the process of becoming a licensed professional engineer (p.e.). The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. Iron has four stable isotopes: It is designed for recent graduates. Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. Iron makes up 5 percent of earth’s crust.
It is designed for recent graduates. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%). The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. Iron makes up 5 percent of earth’s crust. The fe exam is generally your first step in the process of becoming a licensed professional engineer (p.e.). Iron has four stable isotopes: Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take.
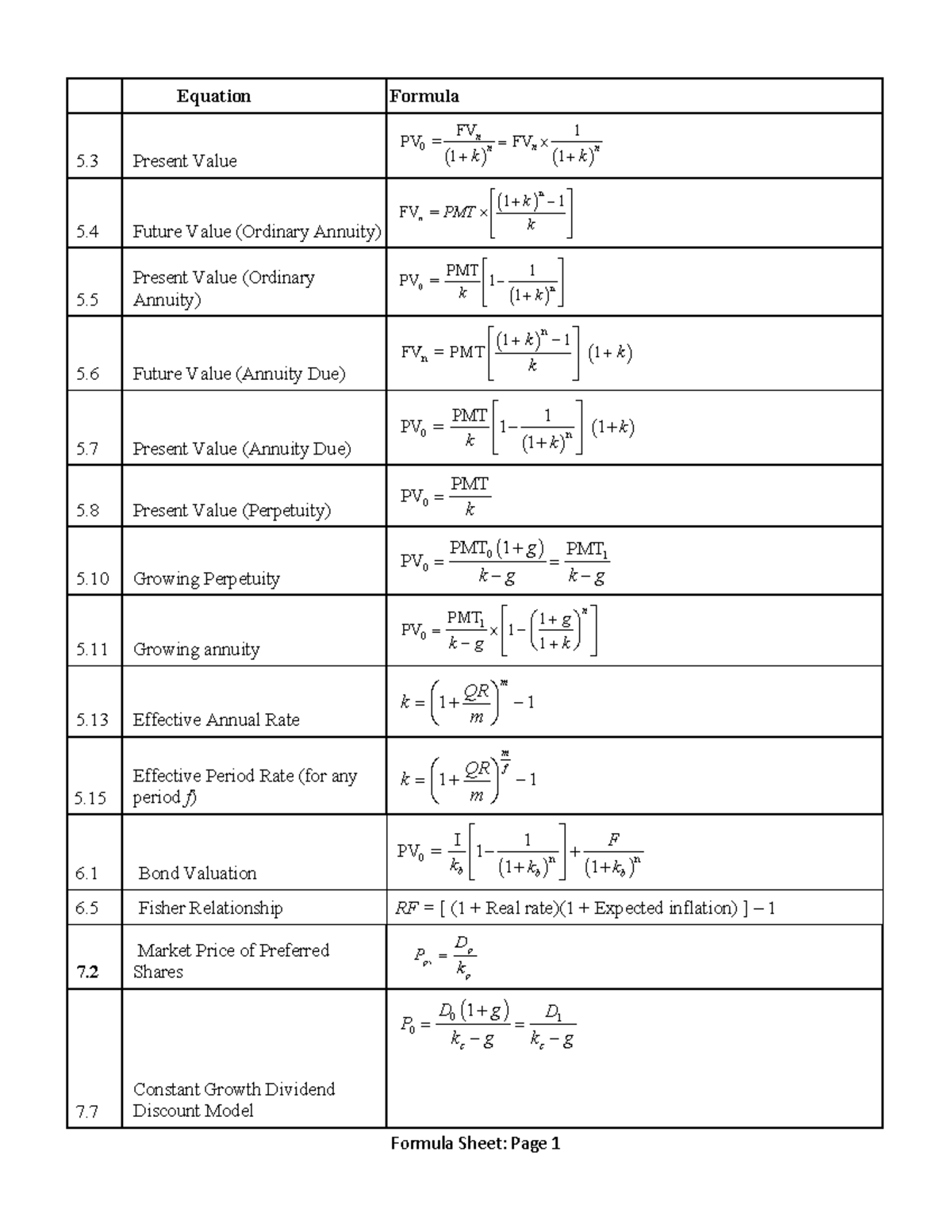
Final Exam Formula sheet Formula Sheet Page 1
The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. The fe exam is generally your first step in the process of becoming a licensed professional engineer (p.e.). It is designed for recent graduates. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe.
Formula Sheet For Fe Exam at Christy Carter blog
Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%). The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. Iron.
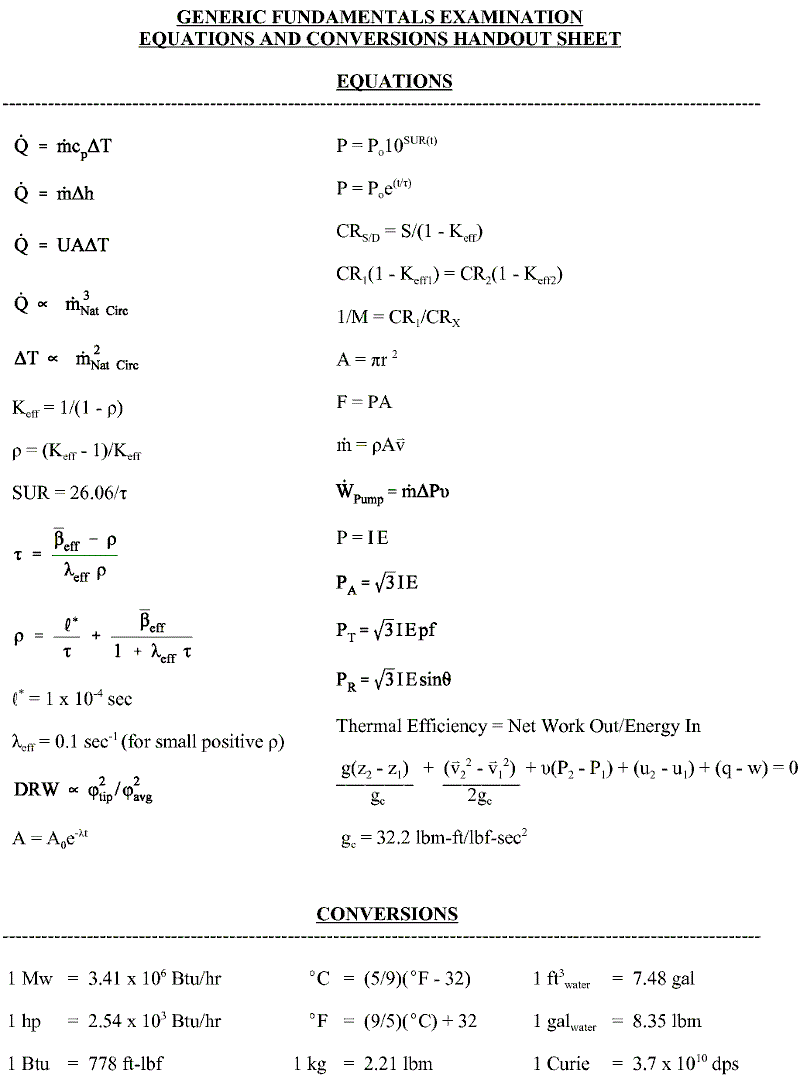
Ultimate FE Reference Handbook Guide. Master these and Save Valuable
Iron makes up 5 percent of earth’s crust. The fe exam is generally your first step in the process of becoming a licensed professional engineer (p.e.). The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and.
Chemical Engineering Section of The FE Chemical Engineering
Iron has four stable isotopes: The fe exam is generally your first step in the process of becoming a licensed professional engineer (p.e.). Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe.
Formula Sheet For Fe Exam at Christy Carter blog
54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%). Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. It is designed for recent graduates. Iron has four stable isotopes: Iron makes up 5 percent of earth’s crust.
Dynamics Equation Sheet KINEMATICS Particle Rectilinear Motion
The fe exam is generally your first step in the process of becoming a licensed professional engineer (p.e.). Iron has four stable isotopes: Iron makes up 5 percent of earth’s crust. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%). It is designed for recent graduates.
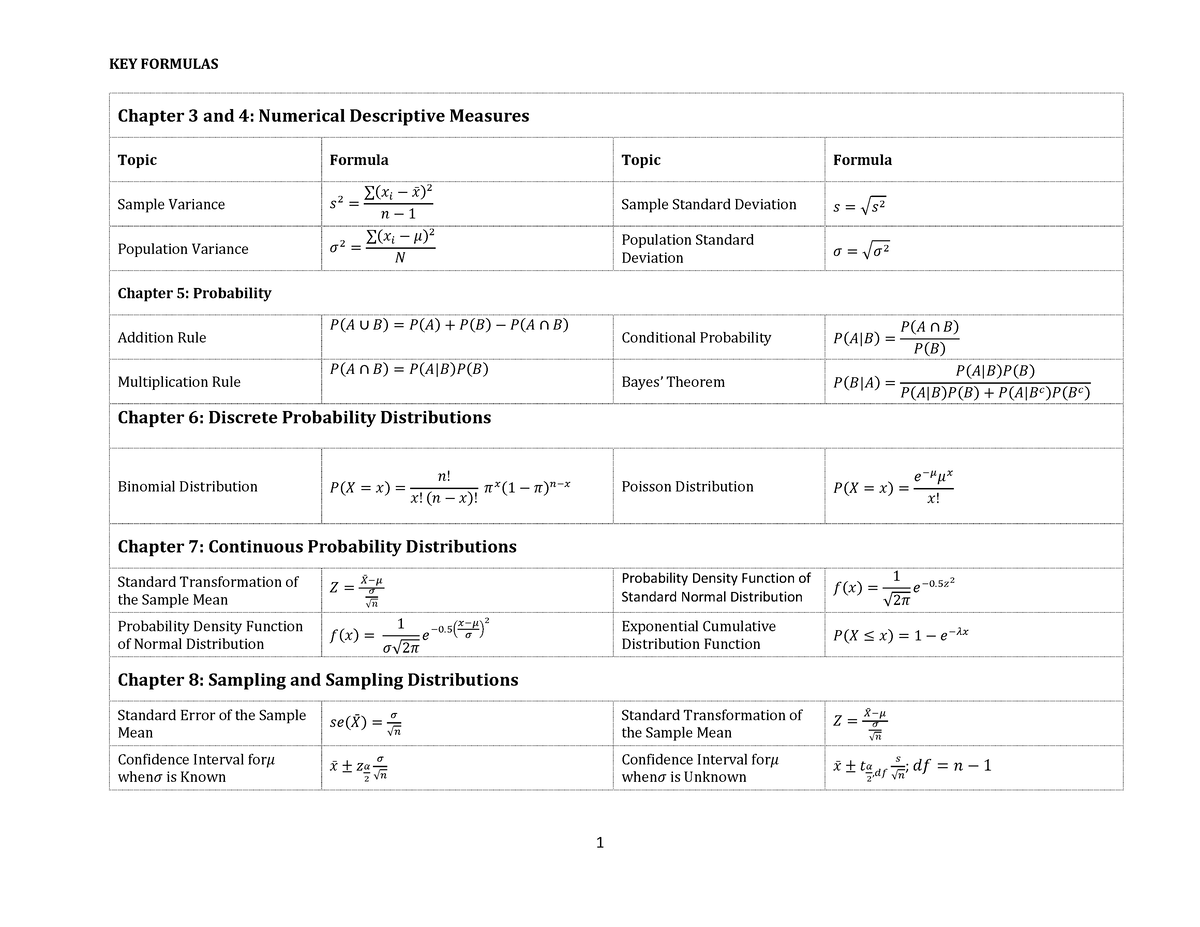
Statistics Formula Sheet for Final Exam
Iron has four stable isotopes: The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%).
Formula Sheet For Fe Exam at Christy Carter blog
Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. Iron makes up 5 percent of earth’s crust. It is designed for recent graduates. The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. The fe exam.
When Should You Take The FE Exam? Genie Prep
Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. Iron makes up 5 percent of earth’s crust. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%). The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers.
Formula Sheet For Fe Exam at Christy Carter blog
Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. Iron makes up 5 percent of earth’s crust. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%). Iron has four stable isotopes: It is designed for recent graduates.
Iron Makes Up 5 Percent Of Earth’s Crust.
Iron is a mineral in your body that comes from foods like red meat and fortified cereals or from supplements you take. 54 fe (5.845% of natural iron), 56 fe (91.754%), 57 fe (2.119%) and 58 fe (0.282%). The fundamentals of engineering (fe) exam is a crucial first step for aspiring engineers seeking to earn a professional engineering license. It is designed for recent graduates.
The Fe Exam Is Generally Your First Step In The Process Of Becoming A Licensed Professional Engineer (P.e.).
Iron has four stable isotopes: