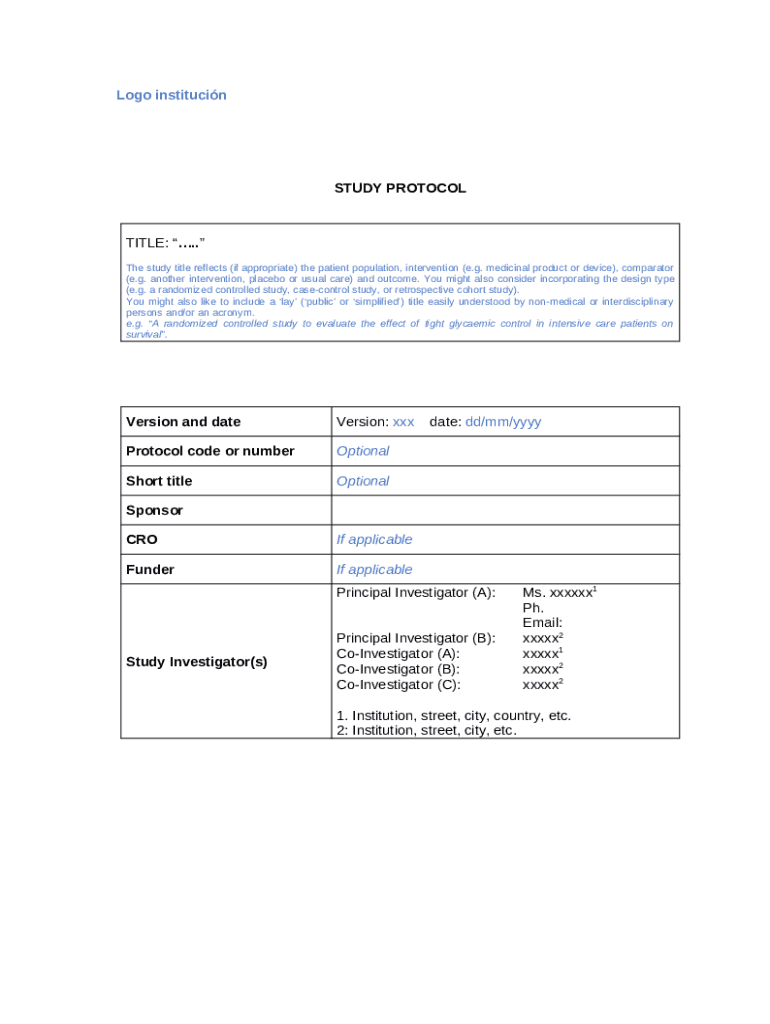
Research Protocol Template - The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. If chart review, indicate whether or not the materials will be obtained prospectively, or if the materials will come from previously existing specimens,. This page includes seven different protocol templates for developing a variety of different new research protocols. A template for randomised trials that includes all 51 spirit headings and item identifiers within the protocol itself. Expedited and full board) human subjects research on this page. Download the template and learn. A guide to prepare a research protocol for health research projects, including sections on study title, aims, objectives, hypotheses, design, population,. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated.
This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. A guide to prepare a research protocol for health research projects, including sections on study title, aims, objectives, hypotheses, design, population,. The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. A template for randomised trials that includes all 51 spirit headings and item identifiers within the protocol itself. This page includes seven different protocol templates for developing a variety of different new research protocols. Download the template and learn. If chart review, indicate whether or not the materials will be obtained prospectively, or if the materials will come from previously existing specimens,. Expedited and full board) human subjects research on this page.
The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. A guide to prepare a research protocol for health research projects, including sections on study title, aims, objectives, hypotheses, design, population,. This page includes seven different protocol templates for developing a variety of different new research protocols. A template for randomised trials that includes all 51 spirit headings and item identifiers within the protocol itself. Download the template and learn. Expedited and full board) human subjects research on this page. If chart review, indicate whether or not the materials will be obtained prospectively, or if the materials will come from previously existing specimens,. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated.
Research Protocol Template Doc Template pdfFiller
Download the template and learn. Expedited and full board) human subjects research on this page. The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. If chart review, indicate whether or not the materials will be obtained prospectively, or if the materials will come from previously existing specimens,..
Biomedical Research Protocol Template Investigator Guidance
This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. Download the template and learn. This page includes seven different protocol templates for developing a variety of different new research protocols. Expedited and full board) human subjects research on this page. A template for randomised trials that includes all.
(PDF) Using a protocol template for case study planning
Download the template and learn. Expedited and full board) human subjects research on this page. The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. A guide to prepare a research protocol for health research projects, including sections on study title, aims, objectives, hypotheses, design, population,. A template.
Clinical Trials 03 Powerpoint Template vrogue.co
A template for randomised trials that includes all 51 spirit headings and item identifiers within the protocol itself. This page includes seven different protocol templates for developing a variety of different new research protocols. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. Download the template and learn..
Free Study Protocol Template to Edit Online
Expedited and full board) human subjects research on this page. A template for randomised trials that includes all 51 spirit headings and item identifiers within the protocol itself. This page includes seven different protocol templates for developing a variety of different new research protocols. This protocol template is a tool to facilitate the development of a research study protocol specifically.
Free Clinical Trial Templates Smartsheet
This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. If chart review, indicate whether or not the materials will be obtained prospectively, or if the materials.
Research protocol template
This page includes seven different protocol templates for developing a variety of different new research protocols. A template for randomised trials that includes all 51 spirit headings and item identifiers within the protocol itself. Expedited and full board) human subjects research on this page. Download the template and learn. If chart review, indicate whether or not the materials will be.
Protocol Template Word
If chart review, indicate whether or not the materials will be obtained prospectively, or if the materials will come from previously existing specimens,. Expedited and full board) human subjects research on this page. This page includes seven different protocol templates for developing a variety of different new research protocols. A guide to prepare a research protocol for health research projects,.
research protocol template
If chart review, indicate whether or not the materials will be obtained prospectively, or if the materials will come from previously existing specimens,. Expedited and full board) human subjects research on this page. The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. This page includes seven different.
Clinical Study Protocol PowerPoint and Google Slides Template PPT Slides
This page includes seven different protocol templates for developing a variety of different new research protocols. The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. Download the template and learn. A guide to prepare a research protocol for health research projects, including sections on study title, aims,.
A Template For Randomised Trials That Includes All 51 Spirit Headings And Item Identifiers Within The Protocol Itself.
A guide to prepare a research protocol for health research projects, including sections on study title, aims, objectives, hypotheses, design, population,. Expedited and full board) human subjects research on this page. If chart review, indicate whether or not the materials will be obtained prospectively, or if the materials will come from previously existing specimens,. This page includes seven different protocol templates for developing a variety of different new research protocols.
Download The Template And Learn.
The following guidelines will help you select the most appropriate protocol template for your research project, ensuring it aligns with the specific. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated.