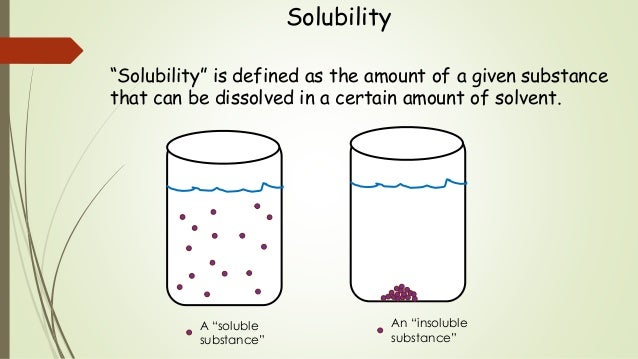
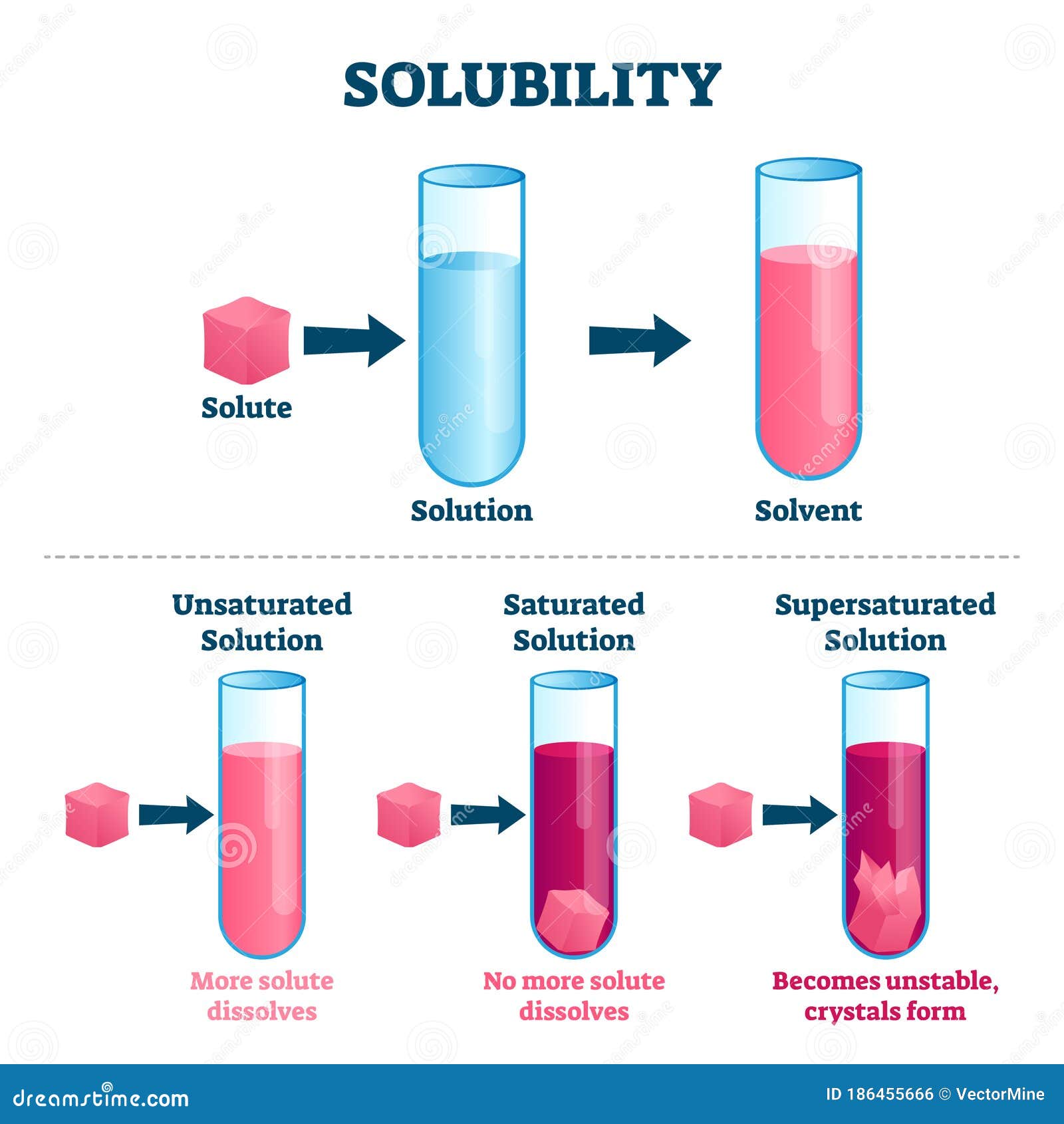
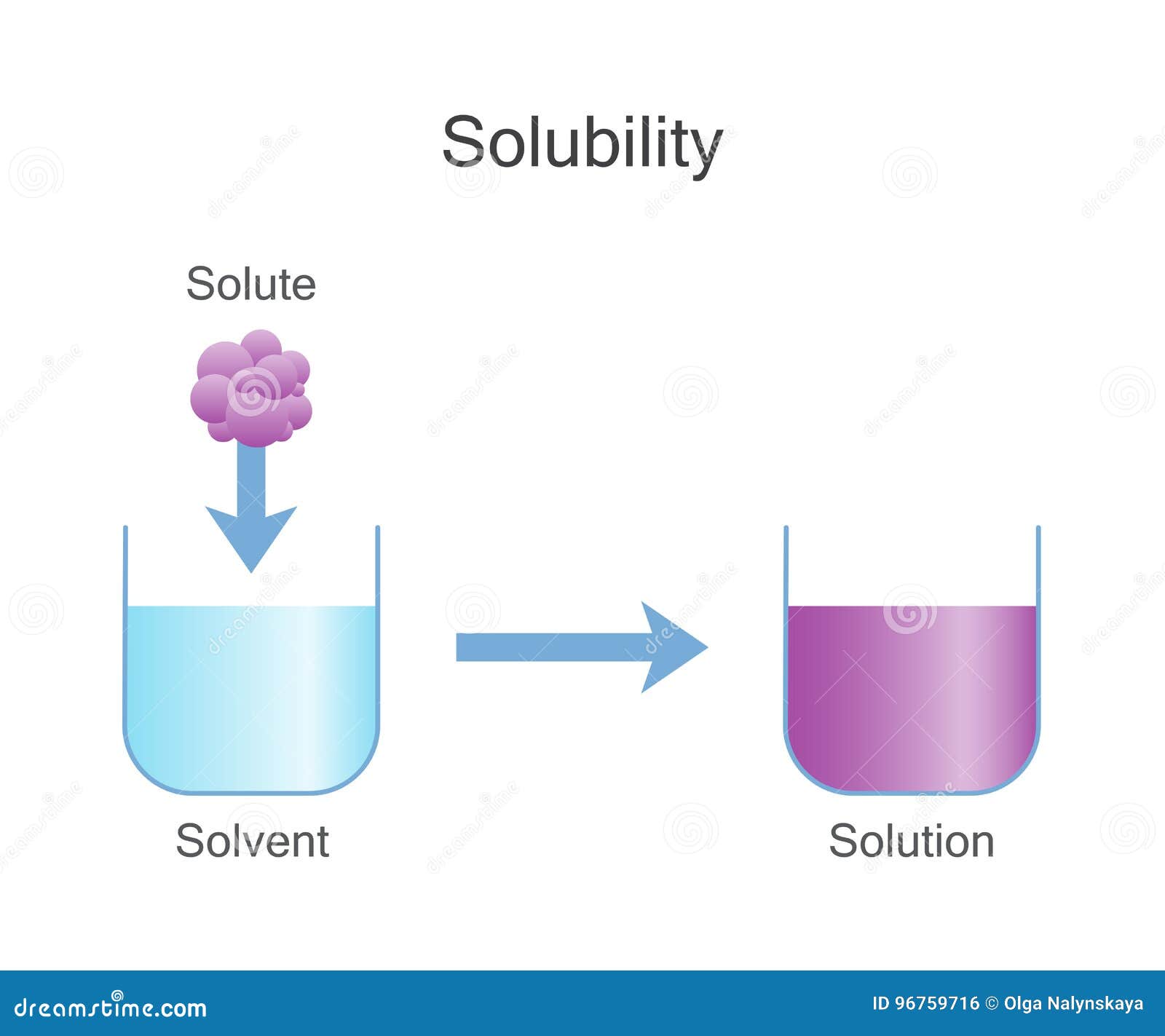
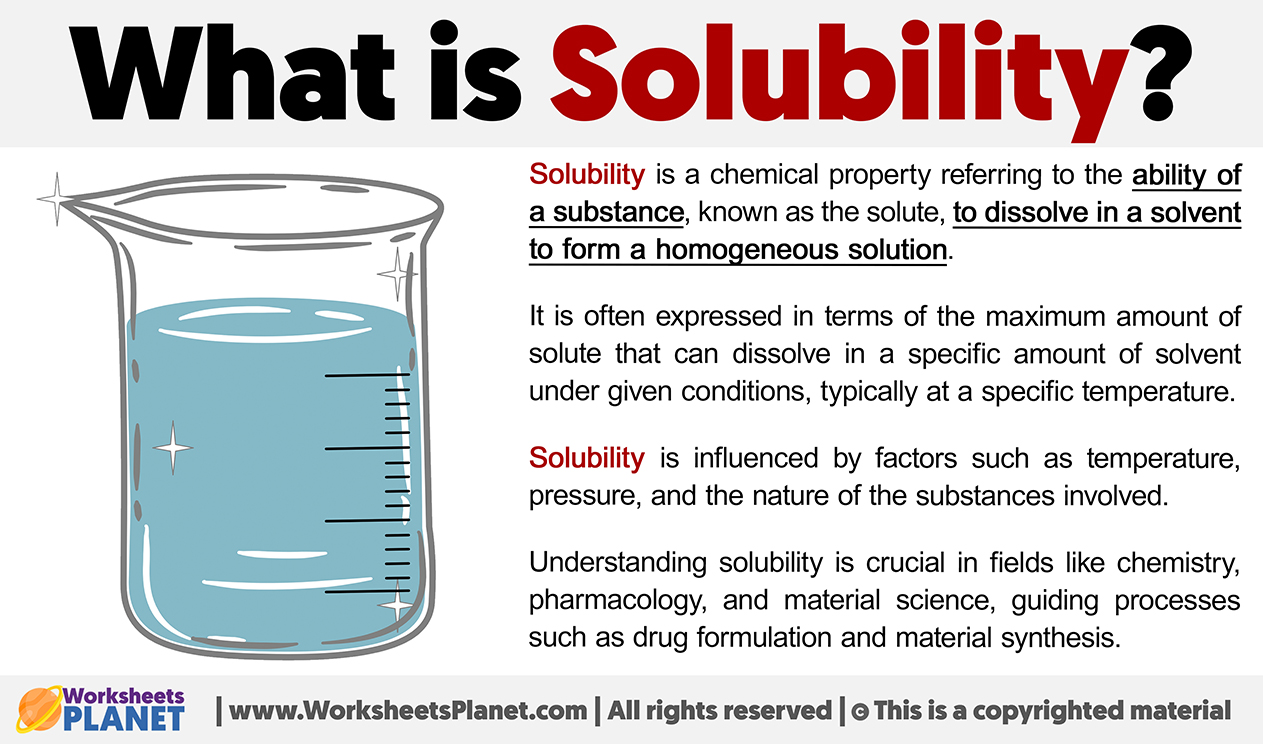
Solubility Sheet - Solubility is the ability of a solute to dissolve in a solvent to form a solution. This is the property that allows things like sugar molecules to dissolve in. In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. The process through which a. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. In such an equilibrium, le chatelier's. Insolubility is the opposite property,. Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent).
This is the property that allows things like sugar molecules to dissolve in. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. Insolubility is the opposite property,. Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent). In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. In such an equilibrium, le chatelier's. The process through which a. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Solubility is the ability of a solute to dissolve in a solvent to form a solution.
Insolubility is the opposite property,. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. In such an equilibrium, le chatelier's. The process through which a. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. This is the property that allows things like sugar molecules to dissolve in. Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent). Solubility is the ability of a solute to dissolve in a solvent to form a solution.
Example Of Soluble
Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent). Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. In chemistry, solubility is the ability of a substance, the solute, to form a.
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. In such an equilibrium, le chatelier's. Insolubility is the opposite property,. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. This is the property that allows things like.
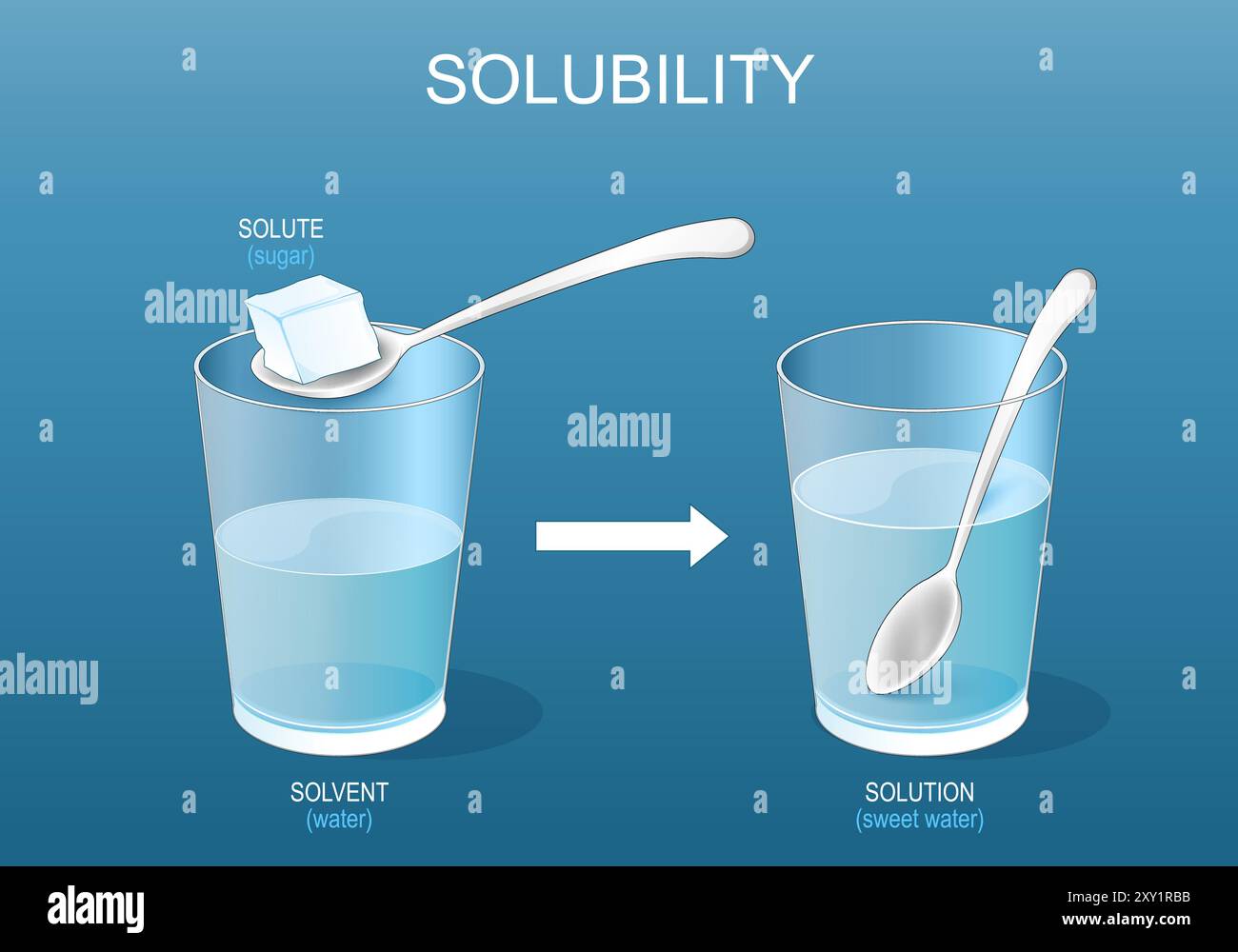
Solubility Vector Illustration. Labeled Solute, Solvent and Solution
Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. In such an equilibrium, le chatelier's. Solubility, degree to which a substance dissolves in a solvent to.
Solid Solubility In Phase Diagrams Solid Solution Phase Diag
The process through which a. Solubility is the ability of a solute to dissolve in a solvent to form a solution. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. In such an equilibrium, le chatelier's. Insolubility is the opposite property,.
Solubility Rules Definition, Examples, and Table
In such an equilibrium, le chatelier's. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. Solubility, degree to which a substance dissolves in a solvent to.
What is Solubility Definition of Solubility
The process through which a. In such an equilibrium, le chatelier's. This is the property that allows things like sugar molecules to dissolve in. Solubility is the ability of a solute to dissolve in a solvent to form a solution. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a.
Solubility General Chemistry Lecture Slides Docsity
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property,. The process through which a. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. In such an equilibrium, le chatelier's.
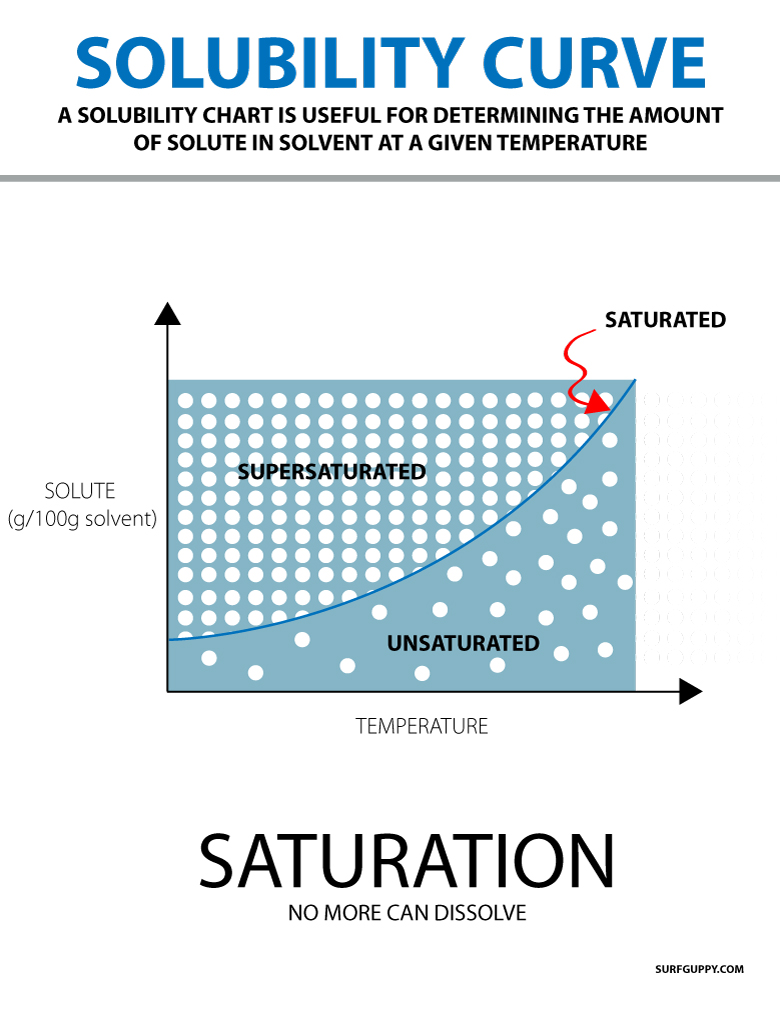
Solubility Surfguppy Chemistry made easy visual learning
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Solubility is the ability of a solute to dissolve in a solvent to form a solution. The process through which a. Insolubility is the opposite property,. This is the property that allows things like sugar molecules to dissolve in.
Dissolution sugar Stock Vector Images Alamy
This is the property that allows things like sugar molecules to dissolve in. The process through which a. In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property,. Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as.
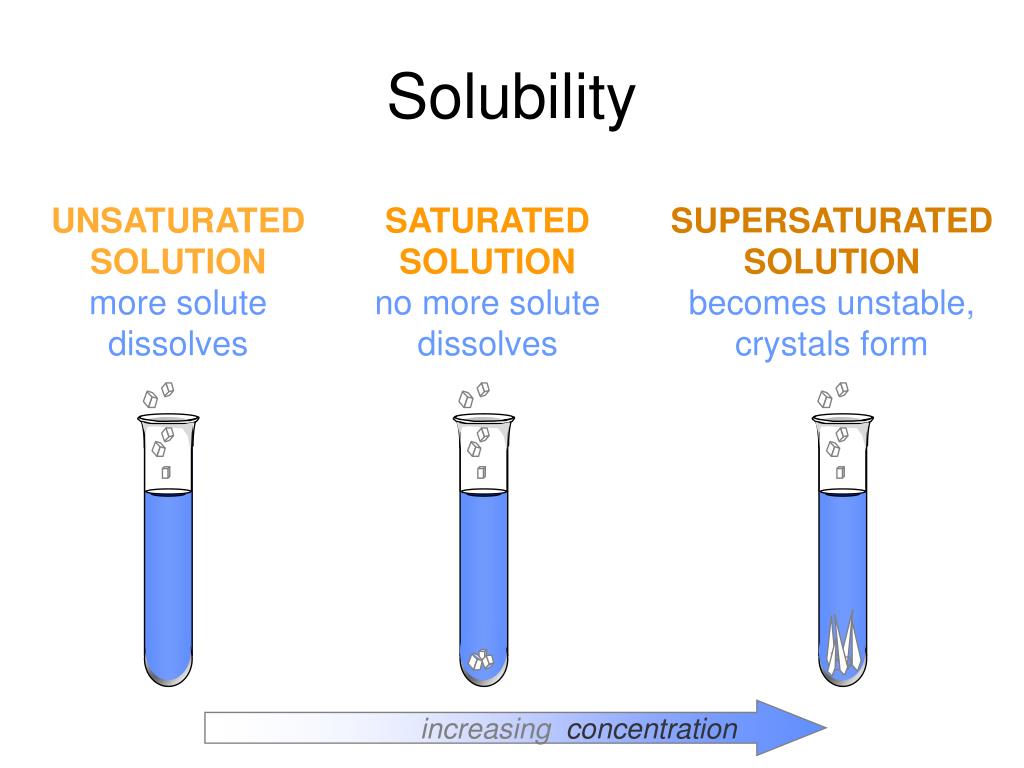
PPT Solubility PowerPoint Presentation, free download ID1115118
Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent). This is the property that allows things like sugar molecules to dissolve in. Solubility.
Solubility, Degree To Which A Substance Dissolves In A Solvent To Make A Solution (Usually Expressed As Grams Of Solute Per Litre Of Solvent).
The process through which a. Solubility is the ability of a solute to dissolve in a solvent to form a solution. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. In such an equilibrium, le chatelier's.
This Is The Property That Allows Things Like Sugar Molecules To Dissolve In.
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property,. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium.