Vis Sheet For Hpv - Explains vis legal requirements, where to find. Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. Access and download current vaccine information statements (viss). You must provide patients with vaccine information statements—it’s the law! Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to.
Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Access and download current vaccine information statements (viss). Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to. Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. Explains vis legal requirements, where to find. You must provide patients with vaccine information statements—it’s the law!
Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to. Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Access and download current vaccine information statements (viss). Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. You must provide patients with vaccine information statements—it’s the law! Explains vis legal requirements, where to find.
HPV (Human Papillomavirus) Immunization Resources for Healthcare
Access and download current vaccine information statements (viss). Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to. You must provide patients with vaccine information statements—it’s the law! Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Prior to the administration of each dose of.
HPV Vaccine CDC
Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Access and download current vaccine information statements (viss). Explains vis legal requirements, where to find. Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to. You must provide patients with vaccine information statements—it’s the law!
Adolescents Riverside University Health System
Access and download current vaccine information statements (viss). Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to. Explains vis legal requirements, where to find. Access the current.
HPV vaccine Fact sheet outlining changes under the National
Access and download current vaccine information statements (viss). Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Explains vis legal requirements, where to find. Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. You must provide patients with vaccine information statements—it’s the law!
Human Papilloma Vaccine Vaccine (HPV) IMC Medical Clinic
Access and download current vaccine information statements (viss). Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. Explains vis legal requirements, where to find. Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Vaccine information statements (viss) are information sheets produced by the cdc that explain.
Human Papillomavirus (HPV) Vaccine Information Statements (VIS
Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to. You must provide patients with vaccine information statements—it’s the law! Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Explains vis legal requirements, where to find. Access and download current vaccine information statements (viss).
Information about HPV Voices For Vaccines
Explains vis legal requirements, where to find. Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Access and download current vaccine information statements (viss). Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. Vaccine information statements (viss) are information sheets produced by the cdc that explain.
Home immunizecanada
Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis). Access and download current vaccine information statements (viss). You must provide patients with vaccine information statements—it’s the law! Explains vis legal requirements, where to find. Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health.
Human Papillomavirus (HPV) Vaccine Information Statements (VIS
You must provide patients with vaccine information statements—it’s the law! Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to. Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. Explains vis legal requirements, where to find. Access.
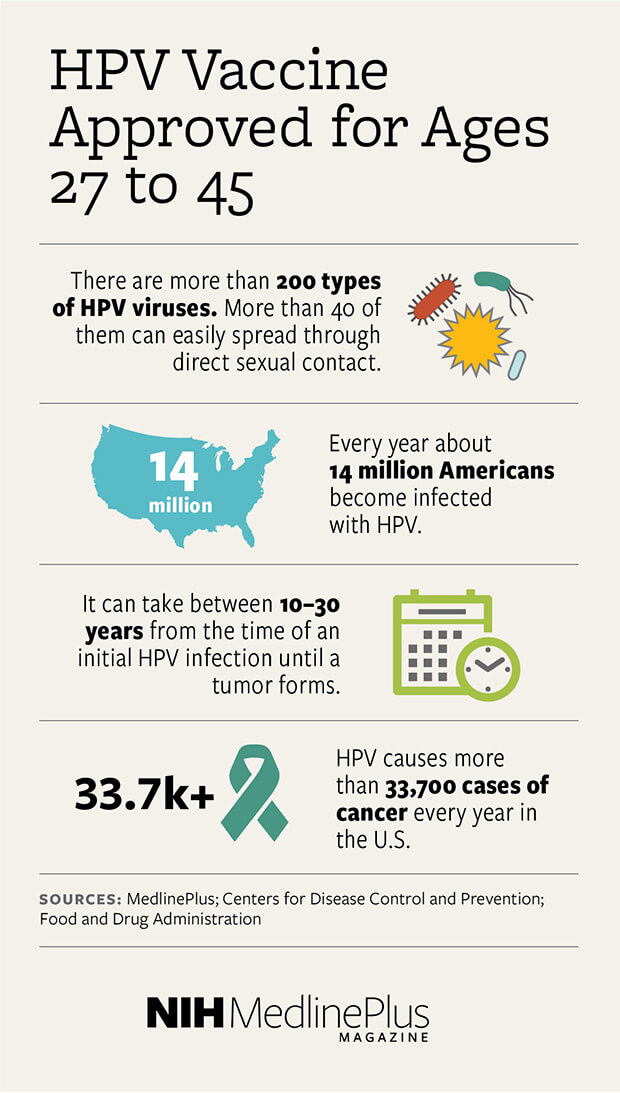
HPV vaccine approved for ages 27 to 45 NIH MedlinePlus Magazine
You must provide patients with vaccine information statements—it’s the law! Vaccine information statements (viss) are information sheets produced by the cdc that explain both the benefits and risks of a vaccine to. Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. Access and download current vaccine information statements (viss)..
Explains Vis Legal Requirements, Where To Find.
Prior to the administration of each dose of vaccine covered under the national childhood vaccine injury act (ncvia), all health. Access and download current vaccine information statements (viss). You must provide patients with vaccine information statements—it’s the law! Access the current influenza (flu) vaccine (inactivated or recombinant) information statement (vis).